According to foreign media reports, scientists have long known that platinum is currently the best catalyst for decomposing water to produce hydrogen. A new study by Brown University researchers in the United States shows why this precious metal is so effective, and the results obtained are not what people think.
(Source: Brown University)
According to the authors of the paper, this research has helped solve a problem that has plagued everyone for nearly a century, and it helps to design new catalysts that are cheaper and richer than platinum to produce hydrogen, which may help reduce fossil fuels. emission.
Andrew Peterson, associate professor of engineering at Brown University and lead author of the study, said: "If we can find a cheap and effective method of hydrogen production, we can open the door to many practical solutions for fossil-free fuels and chemicals. Hydrogen can be used in fuel cells , And fuel cells can be used in hydrogen-powered cars, combined with excess carbon dioxide can make fuel, combined with nitrogen can make ammonia fertilizer, you can use hydrogen to do many things, and if you want to decompose water to produce hydrogen, you need a cheaper catalyst."
Peterson said that the first step in designing a new catalyst is to understand what makes platinum so special in this type of reaction, which is precisely the purpose of this new study.
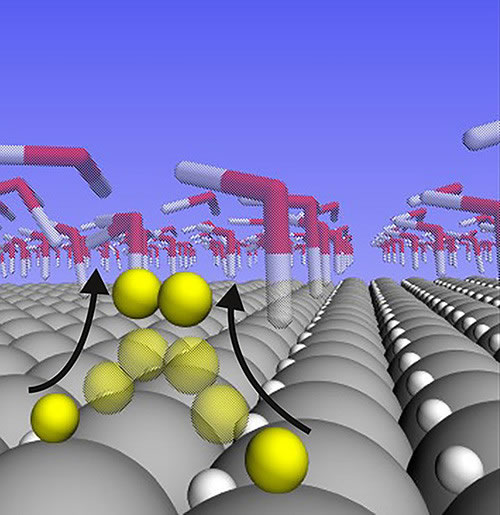
For a long time, platinum has been so successful as a catalyst for the decomposition of water to produce hydrogen because it has the binding energy of the "blonde girl" criterion (just the most suitable). The ideal catalyst needs to be in the reaction so that the molecules are neither too loose nor too compact, but just moderate. If the molecules are too loose, it will be difficult for the reaction to start; if they are too tight, the molecules will stick to the catalyst surface, making the reaction difficult to complete. The binding energy of hydrogen in platinum can just perfectly balance the two parts of the water splitting reaction, so most scientists believe that this is why platinum is a good catalyst.
But Peterson said that there are reasons to question whether this statement is correct. For example, there is a substance called molybdenum disulfide (MoS2) that has a binding energy similar to platinum, but in water decomposition reactions, the catalytic effect is much worse than platinum. Therefore, it shows that the binding energy is not the whole reason.
To find out what happened, Peterson and colleagues studied the performance of platinum catalysts in water splitting reactions. They developed a special method to simulate the behavior of single atoms and electrons in electrochemical reactions.
Analysis shows that when the reaction rate is high, platinum with the binding energy of the "blonde girl" criterion, the hydrogen atoms bound on its surface actually do not participate in the reaction at all. On the contrary, such atoms inhabit the surface crystal layer of platinum and are also inert bystanders (not involved in the reaction). The hydrogen atoms involved in this reaction are much weaker than the assumed binding energy of the "blonde girl" criterion. Such hydrogen atoms do not inhabit the crystal lattice, but sit on top of platinum atoms and can meet freely to form hydrogen gas.
The researchers concluded that it is the free movement of hydrogen atoms on the platinum surface that gives platinum such high activity.
Peterson said: "This tells us that finding the binding energy of the" blonde girl "criterion is not the right principle for designing highly active areas. We suggest designing a catalyst that will allow hydrogen to be highly mobile and active."
Brownson ’s Peterson laboratory specifically used computer simulation to design new catalysts, and the team plans to use the new discoveries to start looking for platinum alternatives. (Author: Yuqiu Yun)
High pressure Globe Valve identified as class 900 and above according to the requirements of ASME B 16.34 which normally used in the high pressure main streaming pipeline or high pressure bypass water feeding pipeline etc. The main function is to cut off/cut on or regulating the medium controlled by electric actuator or handwheel. The sealing principle of pressrue seal seal valve is sealing surface tight off under pressure and they are widely applicated in petrochmical, thermal power plant.
High Pressure Globe Valve,High Pressure Steam Globe Valve,Casted High Pressure Globe Valve,Hydraulic Control Valve
Yongjia South Trading Co.,Ltd , https://www.n-lvalve.com